Myocyte Enhancer Factor 2C
MEF2C (Myocyte Enhancer Factor 2C) is one of four members of the MEF2 family of transcription factors. These are proteins that interact with DNA in specific ways to control the activity and expression of genes. In simple terms, MEF2C is like a regulator, deciding which genes are switched on or off in our cells, which is a fundamental process in our body's development and functioning.
MEF2C is crucial in guiding the development, maturation and differentiation of several types of cells. While most extensively studied in muscle cell development, it is also essential for proper functioning of brain cells (neurons), immune cells (lymphocytes), and cells that form blood vessels (endothelial cells).
The MEF2C protein is structurally complex, comprising several distinct regions known as domains. Each of these domains fulfills a specific role in the protein's function. A pivotal process in the functioning of MEF2C is dimerization, whereby two MEF2 proteins pair up, then bind to DNA and regulate gene expression. This binding to DNA is not random but highly specific, targeting exact sequences to either activate or suppress the reading of genes. The two domains within the protein that are particularly crucial for the dimerization and DNA binding are the MADS and MEF2 domains, respectively. Together, these domains ensure that MEF2C can effectively form pairs and interact with DNA to regulate gene activity.
Beyond its role in gene expression through dimerization and DNA binding, MEF2C also exhibits a remarkable ability to interact with various other proteins across different cell types, helping them assume their specialized functions.
MEF2C gene domains-Courtesy of Biorender
MEF2C Haploinsufficiency Syndrome
In humans, mutations or deletions in a single copy of the MEF2C gene lead to a syndromic form of autism, known as MEF2C Haploinsufficiency syndrome. Affected individuals often exhibit a range of symptoms, including language and social behavior deficits, repetitive motor behaviors, intellectual disability, sensory irregularities, sleep disturbances, seizures, hyperactivity, and motor coordination issues. Although MEF2C plays a crucial role in multiple organ systems, the preponderance of these symptoms are neurological, underscoring its critical function in neurodevelopment.
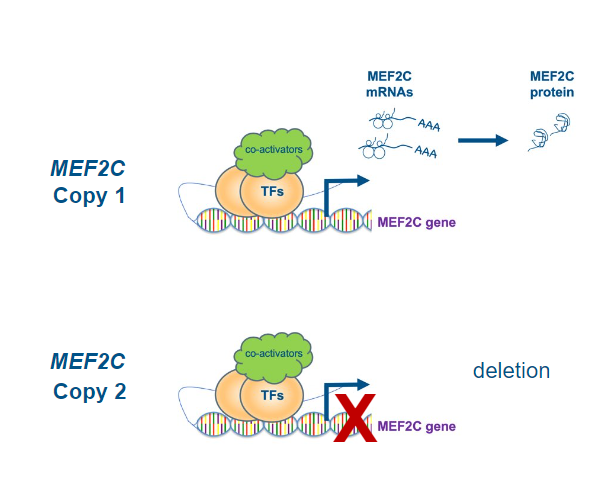
Typically, mutations or deletions in a protein-coding gene can result in various outcomes. Sometimes, the protein may not be produced at all. In other instances, its production might range from being entirely non-functional (loss-of-function) to partially functional, to overly active or even acquiring new functions (gain-of-function). Current understanding suggests that patients with MEF2C Haploinsufficiency syndrome have loss-of-function mutations. The term 'haploinsufficiency' describes a condition where individuals possess only one functional copy of a gene instead of two, leading to insufficient protein levels to maintain a normal phenotype. In fact, many pathological variants of MEF2C are known to occur near or alter the MADS/MEF2 domain, impacting dimerization and/or DNA binding, thereby compromising MEF2C's function as a transcription factor and producing a nonfunctional protein. While this represents our current knowledge of the disease, it's important to acknowledge emerging evidence indicating other mutations with possible gain-of-function, potentially extending beyond classic haploinsufficiency.
Finally, although our understanding of the diverse roles MEF2C plays in various systems and its specific mechanisms of action is incomplete, we know what the fundamental issue in MCHS is: a deficiency of functional protein. Consequently, therapeutic strategies should focus on augmenting MEF2C levels, with the goal of curing or at least alleviating the symptoms and improving patients’ quality of life.
MEF2C Foundation Sponsored Research
The MEF2C Foundation offers grants to help enable investigator-led research to broaden our understanding of MEF2C Haploinsufficiency Syndrome (MCHS) and to develop safe and transformative therapies for MCHS. Please note that the MEF2C Foundation does not support indirect costs for grants.
(Coming Soon) Research programs are active and publications from these projects will be available here
See below for a selection of important and influential MEF2C/MCHS research papers.
Important & Influential Papers
Landmark natural history ((PDF) Clinical findings from the landmark MEF2C‐related disorders natural history study (researchgate.net))
MEF2C hypofunction in neuronal and neuroimmune populations produces MEF2C haploinsufficiency syndrome-like behaviors in mice (MEF2C hypofunction in neuronal and neuroimmune populations produces MEF2C haploinsufficiency syndrome-like behaviors in mice - PMC (nih.gov))
NitroSynapsin therapy for a mouse MEF2C haploinsufficiency model of human autism (NitroSynapsin therapy for a mouse MEF2C haploinsufficiency model of human autism | Nature Communications)
Mutational Analysis of the DNA Binding, Dimerization, and Transcriptional Activation Domains of MEF2C (162627.pdf (nih.gov))